Decoding State Vaccination Rates Using Educational Aptitude, Income, and Political Affiliation
Abstract: COVID-19 cost almost 700,000 (seven hundred thousand) deaths in…

Corresponding author’s name and contact information (e-mail address, mailing address, phone number): Azad Kabir, MD, MSPH, ABIM; Department of Research and Innovation; Doctor Ai, LLC; 1120 Beach Blvd, Biloxi; MS 39530; Department of Internal Medicine, Jackson Hospital, Montgomery, Alabama. Email: azad.kabir@gmail.com; Cell: 228-342-6278
Three large randomized clinical trials named the ATTACC, ACTIV-4a, and REMAP-CAP were terminated early as these trials showed use of therapeutic anticoagulation among non-critical COVID-19 patients increased the probability of survival to hospital discharge as well as reduced the need for cardiovascular or respiratory organ support. These clinical trials also showed when a COVID-19 patient presents with a critical stage, therapeutic anticoagulation does not provide any benefit. This study retrospectively evaluated the COVID-19 admission at Jackson Hospital, Alabama, USA from June 15th, 2020, to June 15th, 2021. The study found that anticoagulation doses can be titrated up or down based on D-Dimer trends and many patients do not need therapeutic anticoagulation, rather an intermediate dose (Lovenox 0.5mg/kg subQ BID or higher dose) anticoagulation can be sufficient for those who have a higher risk of bleeding. The author developed the Kabir bleeding risk score-based treatment strategies for COVID-19 patients which can be visited by clicking on the following link: https://patient.ddxrx.com/anticoagulation.php.
Three largest randomized clinical trials named the ATTACC, ACTIV-4a, and REMAP-CAP were terminated early, and investigators reported on August 4th, 2021, that in noncritically ill patients with Covid-19, an initial strategy of therapeutic-dose anticoagulation with heparin increased the probability of survival to hospital discharge with reduced use of cardiovascular or respiratory organ support as compared with usual-care thromboprophylaxis [1]. These three clinical trials also reported that among critically ill patients with COVID-19, an initial strategy of therapeutic-dose anticoagulation with unfractionated or low-molecular-weight heparin was not associated with a greater probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support than was usual-care pharmacologic thromboprophylaxis [2]. The study observed the same findings of patients with COVID-19 who were already on anticoagulation, even before the diagnosis of COVID-19, and published that those patients had almost near zero mortality rates [3]. This research was submitted on August 15th, 2020, in the Journal of Mississippi State Medical Association [3]. These three clinical trials finding validate the author’s observation why patients already on anticoagulation with Apixaban or Rivaroxaban were surviving as the patients were non critically ill or even asymptomatic when they were taking anticoagulation orally.
According to the Johns Hopkins coronavirus resource center, case fatality rates for COVOD-19 is 1.7% in the United States as of August 17th, 2021 [4]. It is standard of care to treat any hospitalized patient with prophylaxis dose of Enoxaparin (40 mg SubQ daily) or Heparin (5000-unit subQ TID) unless there is a contraindication. It is possible that this low dose of anticoagulation did help reduce case fatality rates in the US have one of the lowest mortality rates for COVID-19. Low molecular weight anticoagulants are weight-based drugs, and it is also possible that those had high fatality due to COVID1-9 were obese and the low dose anticoagulation was inadequate to provide any survival benefit. The goal of this pilot study was to evaluate the effect of variable dose of anticoagulants in treating patient with COVID-19.
The method of the study has been published elsewhere [5]. This study retrospectively evaluated the COVID-19 admission at Jackson Hospital, Alabama, USA from June 15th, 2020, to June 15th, 2021. The author’s previous publication did not assess anticoagulants’ variable effects in treating COVID-19 [3]. The goal of the retrospective pilot study was to evaluate the variable effects of different anticoagulants (like Eliquis and different doses of Enoxaparin [40 mg SubQ BID; 0.5 mg/kg SubQ BID; and 1 mg/kg SubQ BID]) to assess the survival benefit of COVID-19 while treated with anticoagulation.
This study evaluated a variable dose of anticoagulation based on trends of d-dimer and found that intermediate or high dose enoxaparin is associated with better patient outcome and frequently patients do not need therapeutic anticoagulation for survival from COVID-19 [5]. The best treatment strategy from the retrospective study was starting hospital COVID-19 floor patients on Enoxaparin 0.5 mg/kg subq twice a day (for non-critical patients) and titrating dose up if the patient’s condition deteriorates or if d-dimer worsened.
These COVID-19 hospital floor patients provided unique experiences because of their early stages of disease presentation compared to patients admitted in the intensive care units. The study observed COVID-19 patients who presented in the late stages of COVID-19, or those who were directly admitted to the intensive care unit due to intubation or BiPAP requirements, were more likely to have poor outcomes compared to patients presenting at an earlier stage in the COVID hospital floor. Due to poor outcomes with therapeutic anticoagulation in the intensive care unit patients, nationwide intensivists were reluctant to adapt therapeutic anticoagulation in treating COVID-19. The dilemma is mostly rooted in anticoagulation related risk of bleeding and poor patient outcome. This biased experience of intensive care unit physicians leads to higher deaths among COVID-19 patients since the beginning of the pandemic.
Those patients who were admitted at the hospital medical floor (non-critically ill) have significant mortality benefit with concomitant use of anticoagulation and intravenous steroids. However, those patients who were treated with anticoagulation in the early stage of COVID-19 and later moved from the hospital floor to the intensive care unit have a higher chance of recovery compared to those who did not receive any anticoagulation prior to their critical care unit admission. The reason behind those failures is that anticoagulation does not dissolve microthrombi (blood clots) rather prevents their further growth. Treating patients with anticoagulation only can benefit when capillaries are still open (not clogged due to microthrombi). Bottom line is that those patients present in hospitals needing nasal cannula oxygen or high flow nasal cannula oxygen and or patients with low or moderately high D-Dimer (less than two-fold higher than the upper limit of normal) usually survive with anticoagulation and steroid combination treatments. The study observed that increasing trends of D-Dimer in any patient were inversely proportional to the survival rates and or clinical recovery from COVID-19 and vice versa. There is a dose-effect relationship between the decreasing trends of D-Dimer with the used anticoagulation dose. That means the quicker recovery is directly proportional to the higher dose of anticoagulation used for treating early-stage COVID-19.
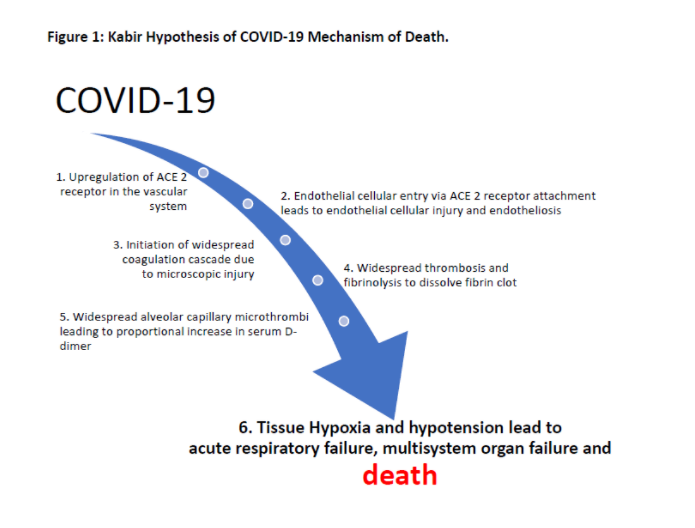
Hence, D-Dimer can be considered to predict patient outcomes, and healthcare facilities’ resources need to tackle COVID-19. Based on the study results, the author developed COVID-19 mechanism of death. Prior to death, patient with COVID-19 develops hypoxia and hypotension (step 6) due to widespread alveolar capillary microthrombi (step 5). The microthrombi load in the body is reflected by increasing trends of D-dimer (step 5 which is been reported in this study) as and it is also responsive to increased dose of anticoagulation. Early initiation of anticoagulation prevents worsening of hypoxia indicates widespread microthrombi formation not happening (step 3) among COVID-19 patients and it also indicates responsiveness to anticoagulation. A previous study already demonstrated that the COVID-19 causes upregulation of the ACE 2 receptor in the vascular system (step 1) [6]. It is a logical next step for COVID-19 to cause endotheliosis while entering endothelial cell by causing endothelial cellular injury (step 2) which leads to widespread coagulation cascade (step 3).
If a state or country’s healthcare system is reaching a breaking point it will be too late for getting vaccination to reduce COVID-19 cases as it takes more than four weeks for the vaccination to start working. In such a case, a contingency plan should be to start a nation or state awareness campaign asking patients to come to seek healthcare at urgent care or primary care clinics so that the chance of capturing early noncritical COVID-19 cases is very high. Again, if patients present to the emergency room in a critical stage, there will not be any benefit of therapeutic anticoagulation. Given the clinical trial [1] findings among noncritical COVID-19 treated with anticoagulation showed reduced use of cardiovascular or respiratory organ support, treating patient at diagnosis with oral therapeutic anticoagulation like Apixaban or Rivaroxaban may reduce need for hospitalization among patient with COVID-19.
Given, all anticoagulant reduces thrombotic risk and at the same time increase the risk of bleeding, the decision to administer an anticoagulant should be based on the assessment of the bleeding risk for the specific patient at a specific point in time. According to the current study, not all patients need therapeutic anticoagulation to treat COVID-19, a lower dose may be sufficient for those with low D-Dimer level. The authors previous study recommends the ideal way to dose anticoagulation is to titrate anticoagulation based on D-Dimer trends [5].
The dilemma of using therapeutic anticoagulation versus the treatment of COVID-19 without anticoagulation to prevent bleeding related mortality and morbidity can be solved if anticoagulation is used judiciously. The author developed the Kabir bleeding risk Score based COVID-19 treatment protocol showed in Table 1. The Kabir bleeding risk calculator can also be visited by clicking the following website: https://patient.ddxrx.com/anticoagulation.php. The author utilized available literature to develop the Kabir bleeding risk calculator [7-9].
If the calculated bleeding score is zero based on Kabir risk score calculator in any patients with COVID-19 or COVID-19 like symptoms, the author suggests starting high dose anticoagulation while titrating anticoagulation dose up or down based on D-Dimer trends (Table 2). However, the author suggests discussion of the risk benefit scenario with patient and family members prior to starting therapeutic anticoagulation among patients with intermediate to high-risk bleeding scores. The author found starting intermediate dose enoxaparin for select patients with high-risk scores is a better strategy than blanket therapeutic anticoagulation in terms of reducing mortality and morbidity related to bleeding associated with anticoagulation.
The author developed the Kabir bleeding risk score-based treatment strategies for COVID-19 patients which can be visited by clicking on the following link: https://patient.ddxrx.com/anticoagulation.php. Mortality and morbidity related to COVID-19 can be further reduced by starting anticoagulation based on Kabir bleeding risk score-based treatments. A reduced dose of anticoagulation is suggested if Kabir bleeding risk score shows intermediate or high-risk of bleeding. In such cases, anticoagulation dose can be titrated up or down based on trends of D-Dimers which will give an opportunity to further reduce bleeding risk among patients who are treated with anticoagulation. Further study is recommended to evaluate whether creating a statewide awareness about getting treatment with anticoagulation as soon as COVID-19 symptoms appears will leads to further decrease in mortality or not.
Acknowledgement: Abul Hussam, Ph.D. inspired me to conduct the research.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance the tenets of the Helsinki Declaration and has been approved by the authors’ institutional review board of Jackson Hospital, Montgomery, Alabama.
Informed consent: All patients were admitted to the hospital as they meet hospitalization criteria and gave signed consent to treatments with any FDA approved drugs.Conflict of Interest: The author has no conflict of interest to disclose.
Table 1: Developing Kabir risk of bleeding score with anticoagulation based on known risk factors: Treating noncritically ill COVID-19 patients.
| RISK FACTORS FOR BLEEDING WITH ANTICOAGULATION | LIKELIHOOD |
| MALE SEX | 0.4 |
| OLDER AGE (≥80; 40 to 80; 18 to 40) | 0.75; 0.5; 0; |
| RACE (ASIAN, HISPANIC, AFRICAN AMERICAN, WHITE) | 1; 0.5; 0.5; 0; |
| CURRENTLY TREATED FOR PEPTIC ULCER DISEASE | 1 |
| BLEEDING EPISODE WITHIN THE PAST 3 MONTHS | 1 |
| PLATELET COUNT <50,000 | 1 |
| ADMISSION IN ICU OR CCU | 0.6 |
| EGFR <30; 30 TO 59; ≥60 | 0.5; 0.4; 0; |
| INR >1.5 | 0.5 |
| ACTIVE CANCER | 0.5 |
| HISTORY OF RHEUMATIC DISEASE | 0.5 |
| PRESENCE OF CENTRAL VENOUS CATHETER | 0.5 |
| PRIOR HISTORY OF STROKE | 0.25 |
| PRIOR HISTORY OF GASTROINTESTINAL BLEEDING | 0.25 |
| RECENT MYOCARDIAL INFARCTION | 0.25 |
| DIABETES MELLITUS TYPE 2 | 0.25 |
| RISK STRATIFICATION | RECOMMENDATION |
| LOW RISK (SCORE 0): | Consider starting high-dose anticoagulation for COVID-19 upon diagnosis. For outpatients, consider therapeutic dose DOAC’s (i.e., Apixaban or Rivaroxaban etc.); For hospitalized patients consider Enoxaparin 0.5 mg/kg or 1 mg/kg dosing based on the initial level of D-Dimer and adjust dose based on D-Dimer trends. |
| INTERMEDIATE RISK (BLEEDING SCORE IN BETWEEN 0 – 2): | Consider starting intermediate-dose or high-dose anticoagulation for COVID-19 upon diagnosis. For outpatients, consider Apixaban 5mg PO BID (adjust based on age or eGFR). For hospitalized patients, consider Enoxaparin 0.5 mg/kg subQ BID and adjust the dose up or down based on D-Dimer trends. |
| HIGH RISK (BLEEDING SCORE > 2): | Consider starting low dose or intermediate-dose anticoagulation for COVID-19 upon diagnosis. For outpatients, consider low dose Apixaban 2.5 mg PO BID. For hospitalized patients, consider Enoxaparin low dose or intermediate dose (40 mg subQ daily or 0.5 mg/kg subQ BID) and adjust the dose up or down based on D-Dimer trends. |
The author developed the Kabir bleeding risk score-based treatment strategies for COVID-19 which can be visited by clicking on the following link: https://patient.ddxrx.com/anticoagulation.php.
Kabir AA. Anticoagulation is the answer in treating noncritical COVID-19 patients. Open Med (Wars). 2021 Oct 5;16(1):1486-1492. doi: 10.1515/med-2021-0354. PMID: 34703901; PMCID: PMC8494147.
Abstract: COVID-19 cost almost 700,000 (seven hundred thousand) deaths in…
Kabir AA. Anticoagulation is the answer in treating noncritical COVID-19…
Discussion about this post